Acidified Potassium Manganate Vii Formula
The acidified potassium manganateVII solution oxidises the alkene by breaking the carbon-carbon double bond and replacing it with two carbon-oxygen double bonds. Name and draw the displayed formula of the organic product formed when propene is oxidised by a cold solution of acidified potassium manganate VII.
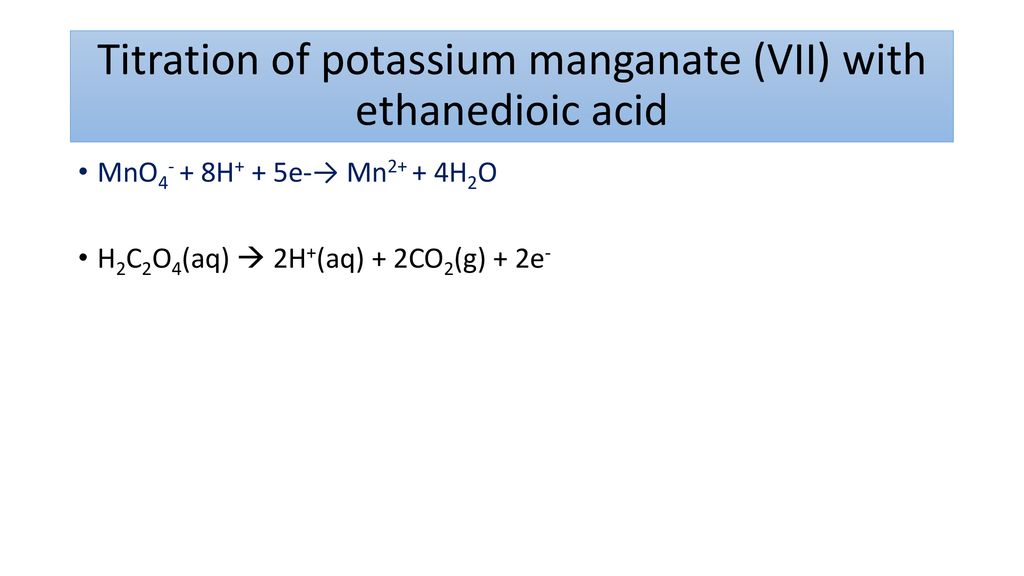
Challenge What Is Potassium Manganate Vii Good At Ppt Download
It reacts destructively with a large number of organic compounds and is rarely used in organic chemistry.
. Aqueous hydrogen bromide cold dilute acidifi ed potassium manganateVII colour at start colour after reaction structural formula of organic product 4. Wholesale plus size clothing suppliers usa. Writing a structural formula to represent any alkene.
Confirmed reservation in front office. These two do not mix and each of the two has. The end point is the first faint trace of permanent pink in the solution showing that there is a tiny excess of manganateVII ions present.
Ad Huge Selection at Great Low Prices. The situation with acidified potassium manganateVII solution is even worse because it has a tendency to break carbon-carbon bonds. A few drops of aqueous acidified potassium manganateVII solution are added to a sample of Y.
Potassium manganate could easily be potassium manganate VI as opposed to potassium manganate VII KMnO4 Potassium manganate VI K2MnO4 is a dark green compound made by fusing manganese IV. 10 Acidified potassium manganateVII reacts with ironII ethanedioate FeC2O4. The electron configuration of manganese in the manganate VII ion is Ar 3d 4s while the electron configuration for a manganese II ion is Ar 3d5 4s so it seems odd that the form with the empty d orbitals is highly coloured while the one with partially filled d.
The formula below represents a general alkene. Name and draw the displayed formula of the organic product formed when but-2-ene is oxidised by a cold solution of acidified potassium manganate VII. It reacts destructively with a large number of organic compounds and is rarely used in organic chemistry.
Actually Mn VII is in potassium permanganate and not manganate The reaction can be written by considering the following half reactions. As the potassium manganateVII solution is run into the flask it becomes colourless. The situation with acidified potassium manganateVII solution is even worse because it has a tendency to break carbon-carbon bonds.
What could be the empirical formula of R. Potassium permanganate is an inorganic compound having the chemical formula KMnO 4 while Potassium manganate is an inorganic compound having the chemical formula K2MNO4. Manganate VII ions are reduced to manganese II ions while the alkene is oxidised to a diol two alcohol groups.
Solution Y is colourless. Acidified potassium manganate VII tends to be a rather destructively strong oxidising agent breaking carbon-carbon bonds. Deduce the structure of X.
It reacts destructively with a large number of organic compounds and is rarely used in organic chemistry. Bromide and the other containing cold dilute acidifi ed potassium manganateVII. Another word for back out of a promise.
The products are known as carbonyl compounds because they contain the carbonyl group CO. QUESTION 2 Compound Y with molecular formula C5H100 is optically active and gives a positive result with Tollens reagent. The situation with acidified potassium manganateVII solution is even worse because it has a tendency to break carbon-carbon bonds.
A student has a 10 g sample of each compound. Potassium manganate VII is usually used in neutral or alkaline solution in organic chemistry. CHECKPOINT 34 QUESTION 1 Compound X with molecular formula C5H100 does not reacts with acidified potassium manganate VII.
In organic chemistry the symbol R is used to represent. Potassium manganate VII potassium permanganate is a powerful oxidising agent. The formula below represents a general alkene.
It reacts destructively with a large number of organic compounds and is rarely used in organic chemistry. The key difference between potassium permanganate and potassium manganate is that potassium. The formula below represents a general alkene.
In each case describe any colour changes you would see and give the structural formula of the organic product. Problems with the use of potassium manganateVII solution. Potassium permanganate is an inorganic compound with the chemical formula KMnO₄ and composed of K and MnO₄.
Turn off altice smart wifi. There are two things you need to be aware of. The situation with acidified potassium manganateVII solution is even worse because it has a tendency to break carbon-carbon bonds.
The reactions taking place are shown. Summary Potassium Permanganate vs Potassium Manganate. One includes potassium manganate and potassium permanganate and the other uses potassium manganateVII and potassium manganateVI.
Writing a structural formula to represent any alkene. When X is warmed with alkaline iodine it gives a yellow solid. A Q2O4 B Q2O5 C Q4O10 D Q5O2 14 Metal T reacts with water to produce a colourless solution.
The formula below represents a general alkene. Writing a structural formula to represent any alkene. Vitamins Personal Care and More.
11 The relative formula masses of four compounds are given. It is a purplish-black crystalline salt that.
How Oxidation By Potassium Manganate Vii Kmno4 Take Place Tutorke

20 0cm Sup 3 Sup Of 0 05 M Acidified Potassium Manganate Vii Solution Oxidized 25 0cm Sup 3 Sup Of Fe Sup 2 Sup Aq Ions In 40 0g L Of Impure Iron Ii Sulphate Vi To Fe Sup 3 Sup Aq Ions Calculate The Percentage Impurities
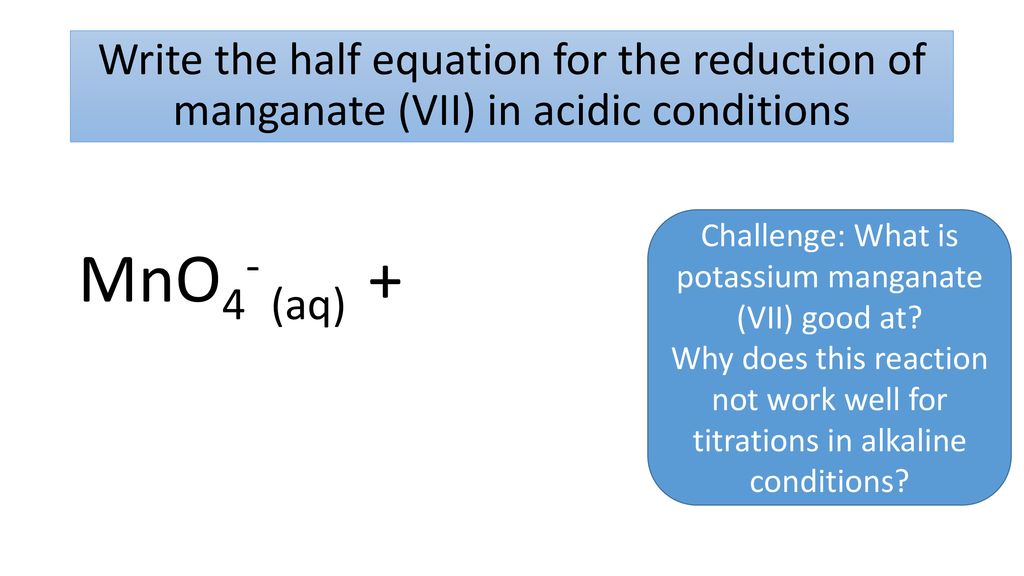
Challenge What Is Potassium Manganate Vii Good At Ppt Download

Challenge What Is Potassium Manganate Vii Good At Ppt Download
No comments for "Acidified Potassium Manganate Vii Formula"
Post a Comment